
Our Research
Biliary function is an essential function of liver, required for the intestinal absorption of fat, cholesterol homeostasis and elimination of diverse metabolic end products. Bile is a vital aqueous secretion of the liver that is formed by hepatocytes and modified downstream by absorptive and secretory properties of the biliary epithelial cells also named cholangiocytes. In homeostatic conditions, both hepatocytes and cholangiocytes are virtually quiescent. However, in case of injury, they proliferate in response to a plethora of factors including growth factors, cytokines, hormones and neurotransmitters and these alterations contribute to liver diseases, which have a major economic impact and account for approximately 15,000 deaths/year in France. In the setting of biliary tract diseases, such as in primary sclerosing cholangitis or cholelithiasis, tissue injury predisposes to biliary-tract carcinomas. Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) account for approximately 10,000 and 4,000 cases/year in France, respectively.
Cancer cell plasticity and cell communication
Epithelial-to-Mesenchymal Transition: Roles of AQP1 & ZEB1 in the tumor progression

Epithelial-mesenchymal transition (EMT) is a multistep process during which epithelial cells gradually adopt structural and functional characteristics of mesenchymal cells. EMT is required for tumor cell migration, invasion and has been considered an early event of metastasis. EMT occurs in cholangiocarcinoma (CCA) where is associated with progression and chemoresistance. The function of aquaporin (AQP) water channels in tumors has been the focus of substantial research efforts. However, the role of AQPs in liver cancers and specifically in CCA remains unknown. Our aim is to study the role of aquaporin-1 (AQP1) in the regulation of EMT in CCA using CCA cell lines KD for AQP1 by CRISPR/Cas9 system.
Likewise, other transcription factors like ZEB1 (Zinc finger E-box binding protein 1) become aberrantly expressed in several cancers both in malignant and stromal cells, e.g., cancer-associated fibroblasts (CAF), tumor-associated macrophages (TAM) and endothelial cells. In pancreatic cancer, which displays a strong desmoplastic stroma, ZEB1 expression in CAF supports cancer progression via paracrine signaling with tumor cells. Similarly, CCA is characterized by a dense desmoplastic stroma produced by myofibroblasts that participate in tumor progression and chemoresistance. In human CCA, ZEB1 expression is associated with poor prognosis. Our aim is to define ZEB1 regulatory functions in malignant and stroma compartments of CCA and to unravel the role of ZEB1 in the chemoresistance mechanisms of stromal CAF.
Key Publication: C Lobe, M Vallette, A Arbelaiz, E Gonzalez-Sanchez, L Izquierdo, A Pellat, N Guedj, C Louis, V Paradis, J M Banales, C Coulouarn, C Housset, J Vaquero, L Fouassier. ZEB1 promotes cholangiocarcinoma progression through tumour dedifferentiation and tumour-stroma paracrine signaling. Hepatology 2021; 74(6):3194-3212
EBP50, a scaffold protein, contributes to hepatobiliary homeostatis and cancer
EBP50 (Ezrin-radixin-moesin (ERM)-Binding Phosphoprotein 50) also named NHERF1 (Na+/H+ Exchanger 3 (NHE3) Regulatory Factor 1 is a scaffold protein that assembles and orchestrates cellular events including the regulation of integral membrane protein activity (transporters, receptors) and signal transduction pathways. By physically tethering proteins, scaffolds locally concentrate, compartmentalize and position transporters/receptors or enzymes in close vicinity of their substrates or regulatory proteins, avoiding background and non-specific interactions. EBP50 is characterized by two protein-protein interacting domains PDZ that bind the C-terminus of target proteins in a sequence-specific manner and an ERM binding domain (EB). Apical EBP50 expression in both hepatocytes and cholangiocytes supports the notion that EBP50 is likely to be involved in bile formation and/or cell proliferation and provide the impetus for studying its function in a more integrative view in the regulation of hepatobiliary homeostasis using EBP50-/- mice.

Key Publications:
- Fouassier L, Yun CC, Fitz JG, Doctor RB. Evidence for ezrin-moesin-radixin-binding phosphoprotein 50 (EBP50) self-association through PDZ-PDZ interactions. J Biol Chem 2000; 275: 25039-45
- Fouassier L, Duan C, Feranchak, AP, Yun CHC, Sutherland E, Simon F, Fitz JG, Doctor RB. ERM binding phosphoprotein 50 (EBP50) is expressed in the apical membrane of bile secretory epithelia. Hepatology 2001; 33: 166-176
- Clapéron A, Guedj N, Mergey M, Vignjevic D, Desbois-Mouthon C, Boissan M, Saubaméa B, Paradis V, Housset C, Fouassier L. Loss of EBP50 stimulates EGFR activity to induce EMT phenotypic features in biliary cancer cells. Oncogene 2012; 31:1376-1388
- Nguyen Ho-Bouldoires TH, Clapéron A, Mergey M, Wendum D, Desbois-Mouthon C, Tahraoui S, Fartoux L, Chettouh H, Merabtene F, Scatton O, Gaestel M, Praz F, Housset C, Fouassier L. Mitogen-activated protein kinase-activated protein kinase 2 mediates resistance to oxidative stress in liver cancer cells. Free Radic Biol Med 2015; 89:34-46
- Vaquero J, Clapéron A, Nguyen Ho-Bouldoires TH, Fouassier L. Role of the PDZ scaffold protein NHERF1/EBP50 in cancer biology: from signaling regulation to clinical relevance. Oncogene 2017;36 :3067-79
Tumor microenvironment and cell signaling
Cholangiocarcinoma-derived extracellular vesicles containing EGFR
Our group has described a major role of epidermal growth factor receptor (EGFR) in CCA development and tumor-stroma crosstalk. EGFR, which is upregulated in CCA, participates in the acquisition of malignant properties by CCA cells and favors tumor growth through the production by stroma of several growth factors and cytokines. Importantly, it has been described that some cancer cells produce extracellular vesicles (EVs) containing EGFR that can be transferred to stromal cells like endothelial cells and stimulate them. It has been postulated that EVs are important mediators in cell-cell communication as signal carriers between cells with relevant roles in the pathophysiology of several diseases including cancer. Interestingly, EVs derived from CCA cells are able to interact with and to activate stromal cells favoring oncogenic processes. Recently, Arbelaiz A et al. (Hepatol 2017 https://doi.org/10.1002/hep.29291) using mass spectrometry-based proteomic analysis, have identified several important oncoproteins, including EGFR, in human CCA cell-derived EVs compared with normal cholangiocyte-derived EVs. We also aim to decipher the role of EGFR activation and CCA-derived extracellular vesicles containing EGFR on the tumor microenvironment.

Key Publications:
- Clapéron A, Guedj N, Mergey M, Vignjevic D, Desbois-Mouthon C, Boissan M, Saubaméa B, Paradis V, Housset C, Fouassier L. Loss of EBP50 stimulates EGFR activity to induce EMT phenotypic features in biliary cancer cells. Oncogene 2012; 31:1376-1388
- Clapéron, M Mergey, TH Nguyen Ho-Bouldoires, D Vignjevic, D Wendum, Y Chrétien, F Merabtene, A Frazao, V Paradis, C Housset, N Guedj, L Fouassier. EGF/EGFR axis contributes to the progression of cholangiocarcinoma through the induction of an epithelial-mesenchymal transition. J Hepatol. 2014 Aug;61(2):325-32
- Pellat A, Vaquero J, Fouassier L. Role of ErbB/HER family of receptor tyrosine kinases in cholangiocyte biology. Hepatology 2017; 67 (2):762-773
- Vaquero J, Lobe C, Tahraoui S, Clapéron A, Mergey M, Merabtene F, Wendum D, Housset C, Coulouarn C, Desbois-Mouthon C, Praz F, Fouassier L. IGF2/IR/IGF1R pathway in tumor cells and myofibroblasts mediates resistance to EGFR inhibition in cholangiocarcinoma. Clin Cancer Res 2018;17: 4282-4296
- J Vaquero, A Pavy, E Gonzalez-Sanchez, M Meredith, A Arbelaiz, L Fouassier. Genetic alterations shaping tumor response to anti-EGFR therapies. Drug Resistance Update 2022;64:100863
Dissecting the tumor microenvironment to improve T-cell infiltration

In the last decade, the immunotherapy age has radically evolved tumor treatments achieving its hopeful bet. However, the expected successes in solid tumors result in a poor clinical response, highlighting the overcome of immunotherapy resistance as an unmet clinical need. Recently, the hype concerning the role of extracellular matrix (ECM) and cancer-associated fibroblasts (CAFs) in the tumor microenvironment (TME) has increased. Cholangiocarcinoma (CCA), a biliary tract cancer, is the prototype of a tumor containing a stiff desmoplastic stroma with a high amount of ECM and CAFs. The stiffness evaluation by shear wave elastography shows that CCA has a high stiffness (57±25kPa) that increases with tumor progression. The tumor dependency on the complex TME suggests how the latter might lead to a resistant tumor status where CAFs could impact drug efficacy by producing an altered ECM. In CCA, FAP-STAT3-CCL2 signaling has been identified in CAFs as sufficient to induce a deleterious inflammatory program by recruiting myeloid-derived suppressor cells (MDSCs) and triggering immunosuppressive activity. In this context, the drop-in of the anti-tumor T-cells is hampered by a stiff TME contributing to immune exclusion. How CAFs, ECM and immune cells interact within TME remains unknown
Antitumoral physical-based innovative therapies: Targeting cancer cells and tumor microenvironment
Cold Atmospheric Plasma as a disruptive approach for the local treatment of Cholangiocarcinoma
As cholangiocarcinoma (CCA) is characterized by a poor prognosis (survival rate <5% at five years) and as the existing treatments remain quite limited, it is mandatory to develop new therapeutic options against CCA, particularly local treatments targeting both the tumor and its microenvironment. In this outlook, cold atmospheric plasma (CAP) shows promises in oncology. Generated from the partial ionization of a gas, CAP generates reactive oxygen and nitrogen species (RONS) that exert deleterious cellular effects leading to cell death or dysfunction. In collaborations with physicists, after setuping a potentially safe atmospheric pressure plasma jet device, we showed using preclinical models of CCA that our apparatus displayed antitumoral effects in both an in vivo and in vitro context. We deciphered the molecular mechanisms underlying these antitumor effects, mainly triggered by an increase in the oxidative state of the tumor cell. We also found out that the CAP treatment was specific to the malignant cells without being deleterious to the surrounding healthy tissues. Now, our aim is to decrypt the effects of cod atmospheric plasma on the tumor microenvironment, especially on the cancer-associated fibroblasts (CAFs) and on the vascularization state of the tumor. Using an immunocompetent murine model of CCA, we also aim to study the effects of our treatment on the immune compartment of the tumor, as recent publications showed that an immunogenic cell death of the tumor cells was triggered after a CAP exposure. In parallel, in close collaboration with clinicians, feasibility and safety studies of the endoscopic plasma probe in pigs are in progress in order to strenghten the use of CAP for the local treatment of CCA via an endoscopic way. The device has already been patented by our lab (Plasma catheter device for conventional endoscopic device, application number: PCT/FR2021/000041).

Key Publications:
- F Judée, J Vaquero, S Guégan, L Fouassier, T Dufour, T. Atmospheric pressure plasma jets applied to cancerology: Correlating electrical configuration with in vivo toxicity and therapeutic efficiency. J. Phys. D Appl. Phys. 2019, 52, 245201
- J Vaquero, F Judée, M Vallette, H Decauchy, A Arbelaiz, L Aoudjehane, O Scatton, E Gonzalez-Sanchez, F Merabtene, J Augustin, C Housset, T Dufour, L Fouassier. Cold-Atmospheric Plasma Induces Tumor Cell Death in Preclinical In Vivo and In Vitro Models of Human Cholangiocarcinoma. Cancers 2020, 12, 1280
- H Decauchy, A Pavy, M Camus, L Fouassier, T Dufour. Cold plasma endoscopy applied to biliary ducts: feasibility risk assessment on human-like and porcine models for the treatment of cholangiocarcinoma. J. Phys. D Appl. Phys 2022, 55, 455401
Video:
Targeting cholangiocarcinoma microenvironment by nanohyperthermia to reduce stiffness and promote immunotherapy strategies
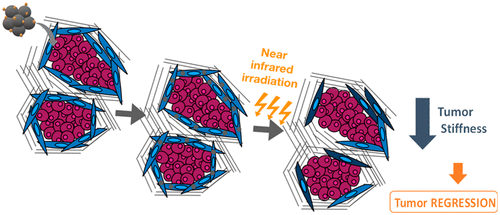
An emerging hypothesis is that the aberrant extracellular matrix (ECM) and anomalous mechanical properties in desmoplastic tumors can decrease not only the ability of chemotherapy and antibodies to penetrate the tumor, but also physically impede immune cells to efficiently reach their target and therefore produce their antitumoral functions. Hence tumor mechanics could play in key role in resistance to immunotherapy in solid desmoplastic tumors. Cholangiocarcinoma (CCA) displays a copious desmoplastic reaction and abnormal mechanical properties directed by the cross-talk between cancer and stromal cells mediated by EGFR signaling and cancer-associated fibroblasts (CAF) activation. We hypothesize that these intrinsic properties confer resistance to anti-cancer treatments that could be lifted by a controlled destruction of the stroma in order to facilitate access of tumor cells to both drugs, antibodies and immune cells. Our aim here is to gather cutting edge technologies to propose a new physical therapeutic approach to modulate the tumor microenvironment (TME) and improve the efficacy of therapies including immunotherapy, mainly by nanohyperthermia in vivo in a murine model of CCA. The first objective of nanohyperthermia is to modulate the TME (CAF depletion and ECM destructuration) and normalize its mechanics (stiffness). As second objective, we will investigate if the modulation of TME promotes inflammatory immune infiltrate and facilitate T-cell ability to contact malignant cells. Since treatment efficacy in CCA is a constant challenge for clinicians, the vision of targeting TME is a new open field that could be considered to reduce treatment resistance. Our third objective is thus to assess the synergy of nanohyperthermia to favor the infiltration of anti-EGFR therapeutic antibody and efficacy of immune checkpoint inhibitor anti-PD-1.
Key Publications:
- AK Silva, A Nicolas-Boluda, L Fouassier, F Gazeau. Overcoming the tumor microenvironment: the role of nanohyperthermia. Nanomedicine (Lond). 2017 Jun;12(11):1213-1215
- A Nicolás-Boluda, J Vaquero, G Laurent, G Renault, R Bazzi, E Donnadieu, S Roux, L Fouassier*, F Gazeau*. Phototermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. ACS Nano 2020 14 (5), 5738-5753
- A Nicolas-Boluda, J Vaquero, L Vimeux, T Guilbert, S Barrin, C Kantari-Mimoun, M Ponzo, G Renault, P Deptula, K Pogoda, R Bucki, I Cascone, J Courty, L Fouassier, F Gazeau, E Donnadieu. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Elife. 2021 Jun 9;10:e58688
